BIOMEDICAL KNOWLEDGE CENTER
- Home
- BIOMEDICAL KNOWLEDGE CENTER
Over the past decade, the medical device and biomaterials industries have undergone tremendous amounts of innovation and change. Materials and technologies have evolved to the point where stents weighing only a few grams can now be surgically implanted into a patient’s neurovascular system, and innovations to packaging are simplifying the drug-delivery process.
Correspondingly, there have been substantial increases in regulatory involvement to ensure safety and efficacy in all products. These evolutions have translated into a growing requirement for the full evaluation of each device from concept to finished product. Physical testing and Finite Element Analysis (FEA) have become commonplace among the research arms of medical device manufacturers around the world. As a total solution provider, Instron® designs and manufacturers both customized and standard systems for testing medical devices and biomaterials.

TRENDING | PPE TESTING
KEY CONSIDERATIONS
When Selecting a Testing System for Biomedical Applications


Reliability
The average life of an Instron system is over two decades, ensuring our customers can be confident in their investment.

Repeatability
Instron designs, manufactures, calibrates, and verifies our own sensors from force transducers to extensometers, ensuring optimal system performance.

Ease of Use
Instron customers trust that any new system is intuitive and easy to use for an operator of all skill levels for any application.

FDA Compliance
Instron offers validation assistance to meet FDA guidelines such as IQ, OQ, and PQ, and offers software that enables users to meet requirements of FDA 21 CFR Part 11.

Application Expertise
With dozens of members on standards committees such as ASTM and ISO, Instron stays at the forefront of application expertise.

Footprint
Bluehill Universal equipped with the Operator Dashboard allows for a small physical footprint on an Instron system. This small footprint allows labs to save space, stay organized, and keep clean.

COMMON APPLICATIONS
- Pharmaceuticals
- Surgical Products
- Orthopedics
- Implantable Devices
- Biomaterials
- Dental
Pharmaceuticals
Materials used in the pharmaceutical industry that require mechanical testing are typically drug delivery systems, tablets, packaging, injectors, luer locks, and syringes. This broad category of materials are most commonly thought of as a means to deliver a drug to a patient. The delivery could be via a shot in a patient’s leg, an intravenous line placed in a patient’s arm, or simply by allowing that patient to open up a medicine bottle. Regardless of the delivery method, it is critical for companies to test the materials and components used in pharmaceutical products to help ensure proper dose, delivery times, patient usability, and safety.
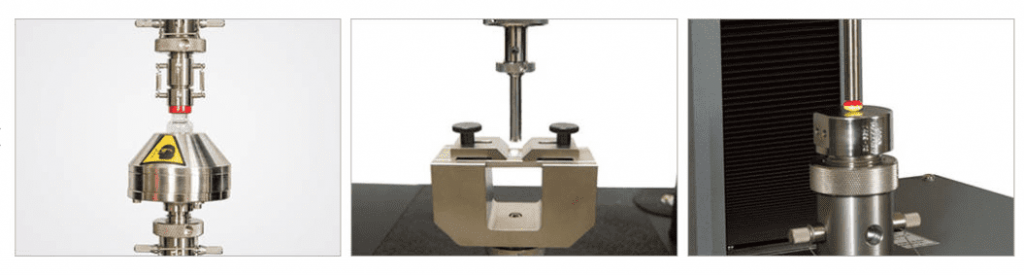
Surgical Products
Surgical products are also known as medical consumables and are the products that are often single-use items found in doctor’s offices and hospitals. These products are often made from plastic materials and include medical tubing, catheters, hospital gowns, gloves, and sutures. These products can range from Class I to Class II medical devices and require a variety of different static mechanical testing.
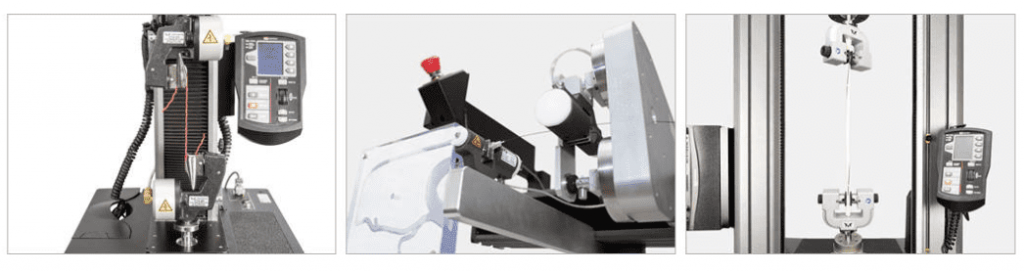
Orthopedics
Orthopedic implants are considered to be specialized implantable devices. Orthopedic implants are implants that support the skeletal system and include bone screws, plates, rods and pins for fracture repair, as well as entire artificial hips, knees, and spinal components. Orthopedic implants can be inserted in the body to assists a patient’s healing and be temporary, or can be inserted in the body with the intent that the implant will outlive the patient. Depending on the use, orthopedic implants are typically considered to be Class II or Class III medical devices by the FDA and require a range and combination of static and fatigue mechanical testing.
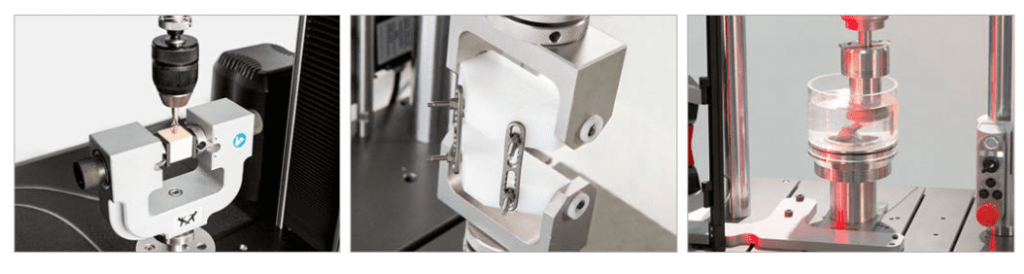
Implantable Devices
Non-orthopedic implantable devices are considered to be permanent implantable devices that do no support the skeletal system. Commonly, implantable devices include medical devices that support the circulatory system and cardiovascular system. For example, stents, heart valves, and pacemakers fall into this category. Implantable medical device are often considered to be classified as Class III medical devices by the FDA, and must undergo stringent mechanical testing through a combination of both static and fatigue testing, before being released to the market.

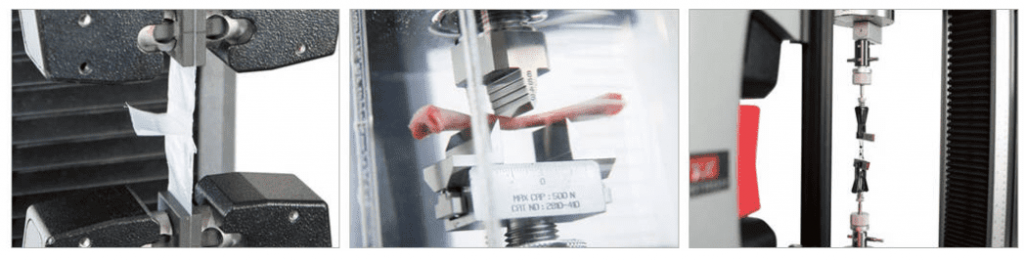
Biomaterials
Biomaterials are the materials found in nature, the human body, and other animal species. These materials can be hard tissues like bone or dental enamel, or soft like tendons and ligaments. As one may expect, biological variation and environmental factors affect the mechanical properties of these materials. These materials are anisotropic and nonhomogeneous making them challenging materials to re-create or engineer outside of nature.
Dental
Dental materials are typically composed of metal, elastomers, and polymers. Restorative and prosthodontic devices are often composed of multiple materials that require mechanical testing to determine how these materials interact to form the entire device. A dental implant is a metal, typically titanium, post that replaces the patient’s entire root and tooth. And artificial tooth, known as a crown, is placed on the dental implant post. Fatigue testing dental implants is the most common mechanical tests in the dental segment.

